Imagine you had a solution of a compound in a good solvent, and you slowly added increasing amounts of a liquid that is not solvent for the compound – what is likely to happen? Unsurprisingly, when some amount of the non-solvent has been added, the compound will no longer dissolve and will start to come out of solution. The now undissolved material could appear as a solid precipitate, or as droplets or a liquid.
A good example of this process is the Greek alcoholic spirit Ouzo. Spirits are typically 40% ethanol (by volume), with the rest being water. Small amounts of the compounds which give the drink it's flavour are dissolved in this ethanol/water mix.
In the case of Ouzo, dissolved trans-anethole contributes to the distinctive aniseed flavour. Trans-anethole is more soluable in ethanol than water and there is just enough ethanol in Ouzo to keep it dissolved. What happens if we add more water (the non-solvent) to the mix? It causes the drink to become cloudy as the oils come out of the solution (the Ouzo Effect)[1,2]. In fact, this is the traditional way to drink Ouzo! The video below by 1univ1 demonstrates the effect perfectly.
Now, imagine that you had a compound that was soluble in both ethanol and water. If you had a solution of this material in ethanol and slowly added water, you would expect it to stay dissolved. Indeed, this is normally the case – nothing precipitates out when you add water to a whisky/whiskey for example.
Adding water to whisky - photo by Lee Carson on Flickr
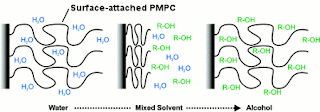
However, with some polymers this is not always the case. For example, a polymer called PMPC (poly(2-(methacryloyloxy)ethyl phosphorylcholine)) dissolves in water, dissolves in ethanol, but does not dissolve in a mix of water and ethanol – strange! This effect is called co-nonsolvency. In fact, many polymers can show co-nonsolvency if you choose the right pair of solvents. For common polymers, these solvent pairs can be quite exotic; polystyrene shows co-nonsolvency in dimethylformamide (DMF) and cyclohexane[3]. PMPC is an exotic polymer, containing a chemical group found in cell membranes called phosphorylcholine, but displays co-nonsolvency in pairs of very common solvents: ethanol and water, and isopropanol and water.
The reasons for co-nonsolvency are
complex, not very well understood, and likely to be slightly
different for different polymers. But it essentially boils down to
this: in the mixed solvents, molecules of the two different solvents
(e.g. ethanol and water) would rather associate with each other than
with the polymer. For something to dissolve, it needs to be
associated with solvent molecules, so the polymer does not dissolve
in the mixed solvent.
In our recent paper[4], we study a layer of PMPC
which has been attached to a surface, rather than free polymer
which can be dissolved or precipitated. Due to the surface
attachment, we have to study co-nonsolvency by watching the thickness
of the layer. When the layer is in a good solvent (pure ethanol or
pure water), it takes up lots of solvent and swells, becoming
thicker. In a bad solvent (the ethanol/water mixes), the PMPC layer
can no longer take up solvent molecules (it does not want to be
swelled by the solvent) and so collapses, becoming thinner. The
layers we're using are very thin, only 30 nanometers (30 millionths
of a millimeter) when dry, and we measure the thickness with a
technique called ellipsometry.
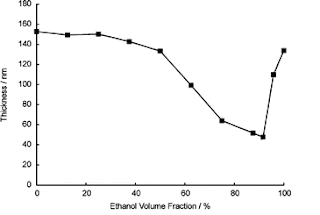
In the graph below, you can see solvent
composition (as percentage of ethanol added to water) plotted
horizontally and the thickness of the PMPC layer plotted vertically.
In the pure solvents (either end of the graph), the layer has swollen
to 140-150 nm from its dry thickness of 30 nm. In the mixed solvent, it
is still slightly swollen but nowhere near as much. This is
co-nonsolvency in action.
Thickness of a surface-attached PMPC layer in ethanol/water mixes
Cartoon showing co-nonsolvency in surface-attached PMPC. R-OH represents alcohol (ethanol or isopropanol)
Why are we interested in PMPC
co-nonsolvency? To be honest, the motivation for this paper was to
check that co-nonsolvency is observed for surface-attached layers in
the same way as free polymer. Polymers are such an important part of
modern life that it is important to understand their properties
fully. PMPC is finding applications in medical devices (as
biocompatible coatings, amongst other applications) and it
will be important to understand the solution properties to allow this
material to be processed and used properly.
Finally, to answer the question posed
in the title: if you are seeing co-nonsolvency, solvents are not
solvents when you mix them together!
References
[1] Liquid Droplet Dispersions Formed by Homogeneous Liquid−Liquid Nucleation: "The Ouzo Effect". Vitale and Katz, Langmuir 2003, 19, 4105.
[2] Spontaneously Formed trans-Anethol/Water/Alcohol Emulsions: Mechanism of Formation and Stability. Sitnikova et al., Langmuir 2005, 21, 7083
[3] Measured and Calculated Solubility of Polymers in Mixed-Solvents - Co-non-solvency. Wolf and Willms, Makromolekulare Chemie - Macromolecular Chemistry and Physics 1978, 179, 2265
[4] Co-nonsolvency effects for surface-initiated poly(2-(methacryloyloxy)ethyl phosphorylcholine) brushes in alcohol/water mixtures. Edmondson et al., Langmuir, 2010, 26, 7216
References
[1] Liquid Droplet Dispersions Formed by Homogeneous Liquid−Liquid Nucleation: "The Ouzo Effect". Vitale and Katz, Langmuir 2003, 19, 4105.
[2] Spontaneously Formed trans-Anethol/Water/Alcohol Emulsions: Mechanism of Formation and Stability. Sitnikova et al., Langmuir 2005, 21, 7083
[3] Measured and Calculated Solubility of Polymers in Mixed-Solvents - Co-non-solvency. Wolf and Willms, Makromolekulare Chemie - Macromolecular Chemistry and Physics 1978, 179, 2265
[4] Co-nonsolvency effects for surface-initiated poly(2-(methacryloyloxy)ethyl phosphorylcholine) brushes in alcohol/water mixtures. Edmondson et al., Langmuir, 2010, 26, 7216
No comments:
Post a Comment